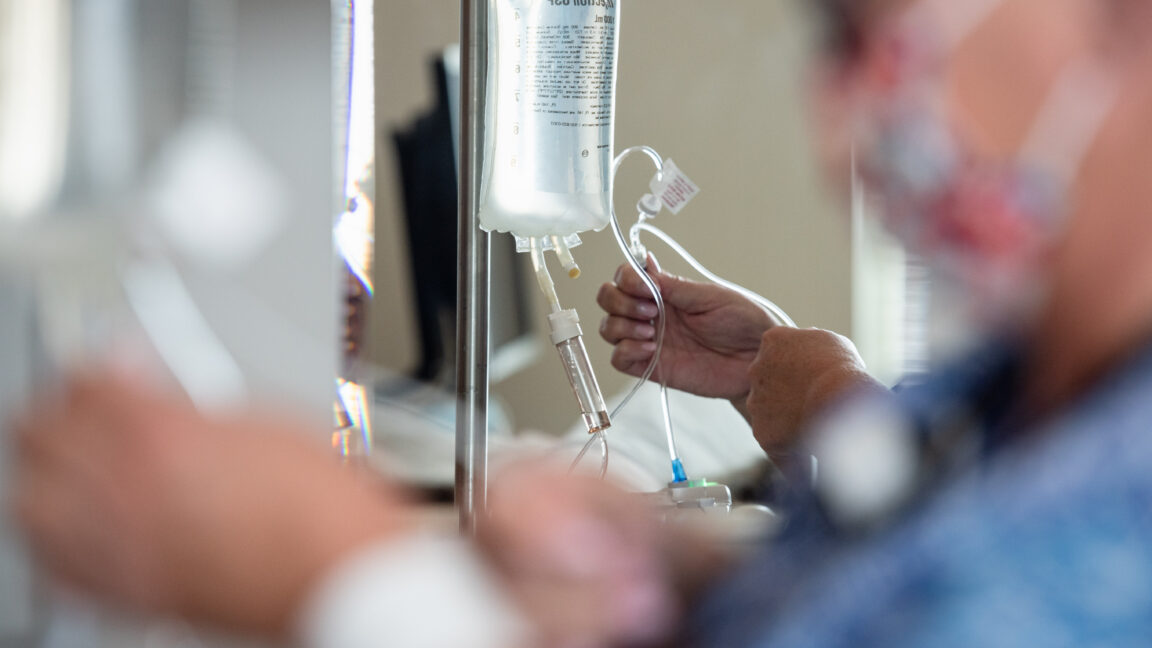
The western North Carolina plant that makes 60 percent of the country's intravenous fluid supply has restarted its highest-producing manufacturing line after being ravaged by flooding brought by Hurricane Helene last month.
While it's an encouraging sign of recovery as hospitals nationwide struggle with shortages of fluids, supply is still likely to remain tight for the coming weeks.
IV fluid-maker Baxter Inc, which runs the Marion plant inundated by Helene, said Thursday that the restarted production line could produce, at peak, 25 percent of the plant's total production and about 50 percent of the plant's production of one-liter IV solutions, the product most commonly used by hospitals and clinics.
“Recovery progress at our North Cove site continues to be very encouraging," Baxter CEO and President José Almeida said. "In a matter of weeks, our team has advanced from the depths of Hurricane Helene’s impact to restarting our highest-throughput manufacturing line. This is a pivotal milestone, but more hard work remains as we work to return the plant to full production."
Overall, Baxter said it is ahead of its previously projected timeline for getting the massive plant back up and running. Previously, the company said it had aimed to produce 90–100 percent of some products by the end of the year. Still, the initial batches now under production are expected to start shipping in late November at the earliest.
One of the many challenges to restoring the facility was the lack of access to the site; Helene had damaged an access bridge. In its latest announcement, Baxter said that a temporary bridge—built with support from North Carolina's Department of Transportation and the federal Administration of Strategic Preparedness and Response (ASPR)—has allowed the transport of more than 885 truckloads of existing inventory out of the plant since Helene. A second temporary bridge, expected to be completed in early November, will enable further access of traffic and equipment to the site.
"Progress at Baxter’s North Cove facility continues to be encouraging," Health Secretary Xavier Becerra said in a statement. "The output from the restarted line, which will be closely monitored to help ensure the quality and safety of released product, will supplement product that is being imported from abroad in accordance with temporary grants of regulatory flexibility from FDA. While important steps remain to ensure the safety of this production, this latest development moves us another step closer to ensuring partners and patients have access to the quality supplies they need."
Since the plant has been shut down, hospitals have hit shortages and order limits of critical IV and dialysis fluids. In one survey, over 86 percent of health care providers said they were affected. In an informational briefing earlier this week, the US Department of Health and Human Services discussed best practices with clinicians on how to conserve IV fluids until the supply is restored. Strategies include giving some patients oral hydration, such as Gatorade or Pedialyte, as well as reducing unnecessary use and changing the way inventory is stored and distributed around health care facilities. Some facilities have also canceled or delayed surgeries and procedures due to the shortage.
ASPR and other federal agencies have taken measures, such as allowing temporary importations, expiration date extensions, and offering compounding guidance to bulk up supply as the Baxter plant recovers.